What is a vector?
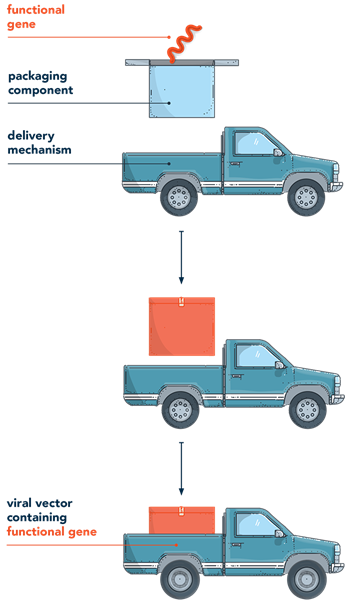
Gene therapy works by introducing genetic material (such as a transgene or nuclease) into the nucleus of the cell. The system used to deliver genetic material is known as a vector. Think of a vector as a microscopic delivery truck that transports packages (genetic material) to specific locations (target cells).
There are two types of vectors, viral and non-viral. Viral vectors are currently a delivery vehicle used in FDA-approved gene therapies. Non-viral techniques are currently being studied as a safe and effective way to deliver genetic material to cells for therapeutic effect.
Gene therapy works by introducing genetic material (such as a transgene or nuclease) into the nucleus of the cell. The system used to deliver genetic material is known as a vector. Think of a vector as a microscopic delivery truck that transports packages (genetic material) to specific locations (target cells).
There are two types of vectors, viral and non-viral. Viral vectors are currently a delivery vehicle used in FDA-approved gene therapies. Non-viral techniques are currently being studied as a safe and effective way to deliver genetic material to cells for therapeutic effect.
Gene therapy works by introducing genetic materialGenetic materialrefers to DNA or RNA that play a fundamental role in creating proteins critical to a cell’s structure or its function in the body
See glossary for more terms > (such as a transgeneTransgenea portion of DNA from one organism inserted into the genome of another organism
See glossary for more terms > or
nucleaseNucleasean enzyme that is capable of cleaving the bond between two bases in a nucleic acid at a specific sequence
See glossary for more terms >) into the nucleus of the cell. The system used to deliver genetic material is known as a vectorVectora delivery system used to introduce genetic material into the nucleus
See glossary for more terms >. Think of a vector as a microscopic delivery truck that transports packages (genetic material) to specific locations (target cells).1-4
Types of vectors
There are 2 types of vectors: viralViral vectora way to deliver genetic material to a cell using the blueprint of a virus as a guide; it may be used to carry genes and change mutated cells to healthy ones
See glossary for more terms > and non-viralNon-viral vectora way to deliver genetic material to a cell that is not based on a virus
See glossary for more terms >. Each type of vector offers different methods of delivering genetic material into cells.1,3,5,6
- Non-viral vectors depend on physical or chemical methods of delivering genetic material into a cell. This can be either a physical technique (like a needle entering a cell) or a chemical technique (created in a lab). The most actively researched non-viral vectors include chemical disruptionChemical disruption vectora type of vector that is typically designed to target specific cells and increase the delivery of genetic material to cytosol or nucleus
See glossary for more terms >, electroporationElectroporationthe use of an electric field to make a cell more permeable, which allows the delivery of genetic material
See glossary for more terms >, and polymer-based vectorsPolymer-based vectorpolymers are one of the substances used to create chemical vectors. These complexes protect DNA and facilitate cell uptake and intracellular delivery
See glossary for more terms >. Non-viral techniques are actively being studied for their safety and efficacy (how well it works)5 - Viral vectors are built using a blueprint of a virus—not the actual virus itself. Scientists only use the parts of the blueprint of the virus that help with delivering genetic material. Currently, viral vectors are the most common vehicle used in FDA-approved gene therapies2
Why are viruses used to deliver gene therapy?
Viruses are used as models or blueprints to create viral vectors. This is because viruses are good at entering the nucleus and delivering instructions to a host cell—much like a delivery truck delivers packages to people. Scientists have actually mapped the complete genetic material (genome) of many viruses. They are able to isolate the helpful elements of a virus’ genome—the delivery truck components—and create them on their own as the starting point for a viral vector. To create a viral vector, only a few parts of the virus are used. These parts alone are not adequate to cause viral infection.
Viruses provide an ideal model for delivering gene therapy to a host cell—the target location where a researcher will want gene therapy to treat a genetic mutation. Because of a virus’ natural design, they are very effective at entering a cell—just like how a delivery truck knows the neighborhood streets so well that it can easily navigate to peoples’ homes and deliver their packages. Scientists have created blueprints, or mapped, the complete genomeGenomethe entire set of genetic instructions found in a cell nucleus
See glossary for more terms > (complete set of genes of an organism) of many viruses. They are able to isolate parts of the virus genome that are effective at entering cells while removing the parts of the virus genome that could cause a disease. Only a few, safe parts of the original virus blueprint are used, and these parts alone are not adequate to cause viral infection.2,3
While a number of viral blueprints exist, the choice of vector is based on characteristics such as duration of gene expressionGene expressionthe information encoded in a gene is used to direct the assembly of a protein molecule
See glossary for more terms >, the size of the genetic material is can deliver, target cells, and immunogenicityImmunogenicitythe degree to which a substance triggers an immune response
See glossary for more terms >.1
Once the vector reaches the target cell, it is able to:
- Pass through the cell membrane, enter the cell, and reach the nucleus2,3
- At the nucleus, it disassembles itself (the package is taken out of the delivery truck)2,3
- Delivers the genetic material (the package) into the nucleus2,3
Now in the nucleus, the genetic material instructs the cell to provide the desired treatment effect. The cell then naturally breaks down the vector and it’s disposed of by the body.7

Can viral vectors cause a viral infection?
In short, no. For a virus to cause a viral infection—an infection caused by a virus—its genome must be nearly complete. When using any virus (adenovirus, adeno-associated virus, or lentivirus) as the basis of a vector for gene therapy, scientists use only certain parts or components of the virus, and they do not use parts of the gene that cause an infection or allow for the virus to replicate itself within the body. In other words, scientists do not use the complete genome of a virus when constructing a viral vector.1,2,4
Before a virus can be used as the basis of a vector, scientists must make a blueprint of the viral genome. In other words, they need to map out every gene that makes up that virus. They use that blueprint to select specific pieces of the virus genes that are needed to deliver therapeutic genetic material to cells (naturally occurring viruses, known as wild-type virusesWild-type Virusa naturally occurring, non-mutated strain of a virus
See glossary for more terms >, are not used to make a vector). The parts of the viral genome that are not needed for delivery of genetic material are never used. This is to ensure that the vector does not produce the infection that would be caused by the complete virus.8-11
 Gene-ius Questions
Gene-ius Questions
Let's use a lentiviral vector as our example. The most well-known lentivirus is the human immunodeficiency virus (HIV). Because HIV is naturally good at entering cells, it’s a perfect candidate to make a vector blueprint from.2,11
The HIV genome is made up of 9 genes and every single gene is required to cause disease. But to make the vector, scientists only select 3 or 4 different genes from the blueprint of the viral genome. Because only 3–4 of the 9 genes from the viral genome are created to make the vector, the genome of the virus is not complete and cannot replicate; hence it will not create the infection caused by the complete virus.1,4,8,11

Viral vectors in gene therapy

Lentiviral vectors (LVVs)
LVVs are a species of retrovirusretrovirusa virus that uses RNA as its genetic material; when a retrovirus infects a host cell, the RNA is converted into DNA, which then incorporates into the genome of the host cell
See glossary for more terms >. The most well-studied lentivirus is HIV, and scientists have used its blueprint to design lentiviral vectors for gene therapy. Lentiviral vectors have the ability to enter the cell and insert its genetic material into dividing cells (such as stem cells) and non-dividing cells (such as cardiac cells). Lentiviruses also integrate genetic material into the host genome. This integration means that the genetic material has increased durability, which allows for continued gene expressionGene expressionthe information encoded in a gene is used to direct the assembly of a protein molecule
See glossary for more terms >.3,12,13
History and areas of research
- HISTORY: this type of vector became routine in research using mammalian cells in the early-1980s. Since then, research advancements have allowed for the development of newer generations of lentiviral vectors, with enhancements that improve the safety and stability of gene expression12,13,14
- EXAMPLE AREAS OF RESEARCH: blood diseases and cancer treatment10,13

Adenoviral vectors (AdVs)
The first viral vector used in gene therapy was based on adenovirus, which is a virus that causes the common cold. Some AdVs were found to trigger strong, potentially dangerous, immune reactions in patients. However, considerable research has helped to understand these immune responses, and AdVs have shown promise in the treatment of cancer. Further research in the use of AdVs is still being explored.3,9,15
History and areas of research
- HISTORY: first used for gene delivery in the mid-1950s13
- EXAMPLE AREAS OF RESEARCH: treating cancer tumors, blood vessel treatments, vaccines16

Adeno-associated viral vectors (AAVs)
Research and creation of adenoviruses led to the discovery of AAVs in the 1960s, and they are considered the standard viral vector used in gene therapy today. These types of vectors are the most studied and most frequently used for gene therapy and are currently involved in a wide variety of clinical trials. There are many different types of AAVs. Each type has a different property allowing them to target different cells, ranging from kidney cells to neurons in the brain.3,10,17
History and areas of research
- HISTORY: discovered in the 1960s with the help of electron microscopy2,16
- EXAMPLE AREAS OF RESEARCH: cystic fibrosis, muscular dystrophy, and several central nervous system (CNS) disorders2
 Gene-ius Questions
Gene-ius Questions
Other than being derived from 2 different viruses, one of the main differences between LVVs and AAVs is how and where their genetic material is delivered.
LVVs are considered integrating vectorsIntegrationthe process where a genetic material moves into the nucleus of a cell and integrates with the host genome, allowing for stable expression
See glossary for more terms >, which means they deliver genetic material that permanently integrates into the DNA of dividing and non-dividing cells. Even if the cells divide, the genetic material does not dilute since it becomes part of the original cell's DNA and every new cell that stems from the original one. This means it is durable and over time it is able to continue offering the same therapeutic benefits as when it was first delivered.10,12
On the other hand, AAVs are non-integrating vectorsNon-integrationthe process where genetic material does not integrate with the host genome and thus has transient expression
See glossary for more terms >. When they deliver genetic material, the therapeutic gene does not permanently integrate into the cell’s DNA. Because of the lack of integration, the treatment effect is diluted as the remaining genetic material is reduced each time the cells divide. In cells that do not divide, the genetic expression remains durable. AAVs are also known to have very low levels of immune responses. There are also different types of AAVs that can infect different kinds of cells. Because of this, AAVs are widely used in gene therapy today to target different diseases based on the types of organ and tissues they affect.10,12
 Gene-ius Questions
Gene-ius Questions
While both AdVs and AAVs are viral vectors that have the ability to affect a broad range of dividing and non-dividing cells in the process of delivering genetic material, there are a few differences between them—mostly around packaging capacity, protein expression levels, onset/duration of gene expression, and immune response.18
AdVs have a larger capacity than AAVs. AdVs also have high levels of protein and transient gene expression compared to AAVs low level of protein expression; however, AAVs have the potential for a long-lasting gene expression. When it comes to onset of expression, AdVs can occur around 16-24 hours after infection. AAVs take considerably more time with up to 3-21 days.18
One of the main benefits to AAVs is that they have very low level of triggering an immune response whereas AdVs typically trigger a high immune response in target cells.18
 Next Level Knowledge
Next Level Knowledge
Vectors used in
gene therapy clinical trials

Non-viral vectors in gene therapy
Non-viral vectors may facilitate long-term expression of therapeutic genetic material, although scientists have yet to prove that they can provide long-term treatment effects. Non-viral vectors have become part of clinical trials and continue to be evaluated for use in gene therapy.20
Below, we share 1 example of each type of non-viral delivery method—1 physical (electroporation) and 1 chemical (nanoparticle-based).
Example of a physical vector: electroporation
Electroporation is a non-viral delivery method being studied to deliver gene therapy in clinical trials. With electroporation, scientists use pulses of electricity to cause temporary pores to form in a cell membrane. These pores enable gene therapy to be delivered into the cell where it can take effect. Though electroporation has been explored in vivo, recent clinical trials deliver gene therapy using electroporation on patient cells outside of the body ex vivo.5,21,22
Scientists continue to explore this and other physical ways to deliver gene therapy, including ballistic DNA (also called a “gene gun,” which uses force), sonoporation (which uses sound), and photoporation (which uses light).20
Example of a chemical vector: lipid nanoparticle
The leading non-viral delivery method uses lipid nanoparticles (LNPs). LNPs encapsulate genetic material so that it can be delivered to target cells. Without a delivery system like an LNP, genetic material degrades quickly and cannot reach target cells. LNPs provide scientists with a way to protect and deliver genetic material for gene therapy in vivo. Because LNPs are quick to manufacture, efficient, and scalable to the size of the material being delivered, they have many potential uses in gene therapy.23
See how vectors deliver genetic material
Other topics you may be interested in:

Keep learning with Genehome
References
1. National Institutes of Health. Genetics Home Reference. Help me understand genetics. Accessed May 3, 2021. https://medlineplus.gov/download/genetics/understanding/primer.pdf 2. Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346-358. 3. STAT Reports. The STAT guide to viral vectors, the linchpin of gene therapy. STAT News; 2019. 4. FDA Commissioner. What is gene therapy? How does it work? US Food and Drug Administration. Accessed July 1, 2021. https://www.fda.gov/consumers/consumer-updates/what-gene-therapy-how-does-it-work 5. Al-Dosari MS, Goa X. Nonviral gene delivery: principle, limitations, and recent progress. AAPS J. 2009;11(4):671-681. 6. Kim TK, Eberwine JH. Mammalian cell transfection: the present and the future. Anal Bioanal Chem. 2010;397(8):3173-3178. 7. National Institutes of Health. Gene therapy. Talking glossary of genetic terms. Accessed July 1, 2021. https://www.genome.gov/genetics-glossary/Gene-Therapy 8. Watts JM, Dang KK, Gorelick RJ, et al. Architecture and secondary structure of an entire HIV-1 RNA genome. Nature. 2009;460(7256):711-716. 9. Wold WSM, Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr Gene Ther. 2013;13(6):421-433. 10. Centers for Disease Control and Prevention. A New Study of Hemophilia Occurrence Finds Many More Cases in the United States. Accessed June 29, 2021. https://www.cdc.gov/ncbddd/hemophilia/features/keyfinding-hemophilia-occurrence-US.html 11. Sheridan C. Gene therapy finds its niche. Nat Biotechnol. 2011;29(2):121-128. 12. Warnock J, Daigre C, Al-Rubeai M. Introduction to viral vectors. In Merten O-W and Al-Rubeai M (eds). Viral vectors for gene therapy: methods and protocols, methods in molecular biology. Springer Science+Business Media; 2011. 13. Durand S, Cimarelli A. The inside out of lentiviral vectors. Viruses. 2011;3(2):132-159. 14. Escors D, Breckpot K. Lentiviral vectors in gene therapy: their current status and future potential. Arch Immunol Ther Exp. 2010;58(2):107-119. 15. Cavazzana-Calvo M, Payen E, Negre O, et al. Transfusion independence of HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467(7313):318-322. 16. Shaw A, Suzuki M. Immunology of adenoviral vectors in cancer therapy. Molecular Therapy. 2019;15:418-429. 17. Crystal R. Adenovirus: the first effective in vivo gene delivery vector. Human gene therapy. 2014;25:3-11. 18. Flotte T. Birth of a new therapeutic platform: 47 years of adeno-associated virus biology from virus discovery to licensed gene therapy. Molecular Therapy. 2013;21(11):1976-1981. 19. Vector Bioloabs. Choosing the right viral vector. https://www.vectorbiolabs.com/adenovirus-vs-aav/ Accessed June 23, 2021. 20. Gene therapy clinical trials worldwide. Journal of Gene Medicine. John Wiley and Sons, Ltd. 2019. https://a873679.fmphost.com/fmi/webd/GTCT. Accessed June 28, 2021. 21. Ramamoorth M, Narvekar A. Non viral vectors in gene therapy – an overview. J Clin Diagn Res. 2015;9(1):GE01-6. 22. Fernandes L. CRISPR to correct gene defect that causes sickle cell disease. UCSF. Accessed May 5, 2021. https://www.ucsf.edu/news/2021/03/420137/uc-consortium-launches-first-clinical-trial-using-crispr-correct-gene-defect 23. Arnold C. What’s new in clinical CRISPR? Nature Medicine. 2021;27:184-185. 24. Cullis P, Hope M. Lipid nanoparticle systems for enabling gene therapies. Molecular Therapy. 2017;25(7):1467-1475.